orbital notation of silver|Iba pa : Tuguegarao The total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in . Tingnan ang higit pa Crucial Memory and SSD upgrades - 100% Compatibility Guaranteed for EMAXX EMAXX - All Models - FREE US Delivery. Crucial Memory and SSD upgrades - 100% Compatibility Guaranteed for EMAXX EMAXX - All Models - FREE US Delivery . laptop or motherboard’s capabilities and what you’re upgrading it to. Additionally, Crucial .Sumiklab ang sunog sa isang residential area sa Commonwealth, Quezon City nitong Biyernes ng umaga. Iniulat sa "Unang Balita" ni James Agustin na nilamon ng malaking apoy ang mga bahay sa isang residential compound sa Halamanan St., Barangay commonwealth. Nagsimula umano ang sunog bago mag-ala siete ng umaga at umabot .
PH0 · orbital notation for all elements
PH1 · orbital notation examples
PH2 · orbital notation chart
PH3 · orbital notation calculator
PH4 · orbital diagram for silver
PH5 · noble gas configuration silver
PH6 · electronic configuration of silver
PH7 · ag orbital diagram
PH8 · Iba pa
Note: The steps to install the 2019, 2016, or 2013 versions of Office Professional Plus, Office Standard, or a stand-alone app such as Word or Project might be different if you got Microsoft 365 through one of the following: Microsoft Workplace Discount Program (formerly known as Home Use Program): If you bought Microsoft 365 for personal use .
orbital notation of silver*******Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the nucleus is called the orbital. The sub-energy levels depend on the azimuthal quantum number. It is expressed by ‘l’. The value of ‘l’ . Tingnan ang higit paThe total number of electrons in silver is forty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in silver in . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paThe ground-state electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. This electron configuration shows that . Tingnan ang higit pa Electron configuration of Silver (Ag) [Kr] 4d 10 5s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1: 2, 8, 18, 18, 1: 48: Electron configuration of Cadmium (Cd) . Silver orbital diagram | Image: Learnool The above orbital diagram shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell . The electron configuration for silver (Ag) is based upon the place meant of silver in the fifth row of the periodic table in the 11th column of the periodic table or the .The p orbitals are px, py, and pz, and if represented on the 2p energy with full orbitals would look like: 2p x 2 2p y 2 2p z 2. The expanded notation for neon (Ne, Z=10) is .The electron configuration of silver is [Kr] 4d10 5s1. Writing the electron configuration for silver involves filling orbitals in the order of increasing energy. The Aufbau principle and .orbital notation of silver Iba paThe electron configuration of silver is [Kr] 4d10 5s1. Writing the electron configuration for silver involves filling orbitals in the order of increasing energy. The Aufbau principle and .
Iba paElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .
The orbitals are filled as described by Hund’s rule: the lowest-energy configuration for an atom with electrons within a set of degenerate orbitals is that having the maximum . Ag: properties of free atoms. Silver atoms have 47 electrons and the shell structure is 2.8.18.18.1. The ground state electron configuration of ground state gaseous .
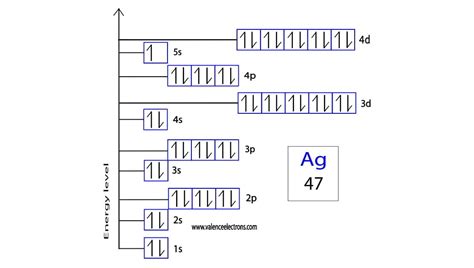
Overview of Silver’s Orbital Diagram. Silver (Ag) is a transition metal that belongs to the d-block of the periodic table. Its electron configuration is [Kr] 4d 10 5s 1, with a total of 47 electrons. To understand the orbital diagram of silver, it is important to understand the basics of orbital notation. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. . Silver (Ag) 48: Cadmium (Cd) 49: Indium (In) 50: Tin (Sn) 51: Antimony (Sb) 52: Tellurium (Te) 53: Iodine (I) 54: Xenon (Xe) 55: Caesium (Cs) .
Electron configuration of Silver (Ag) [Kr] 4d 10 5s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1: 2, 8, 18, 18, 1: 48: Electron configuration of Cadmium (Cd) [Kr] 4d 10 5s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 . Silicon has 14 protons and 14 electrons. the configuration = 1s2 2s2 2p6 3s2 3p2 in the outer orbital, there are 2 electrons, these are shielded from the nucleus by 12 other electrons Sulfer has .Silver. Element 47 of Periodic table is Silver with atomic number 47, atomic weight 107.8682. Silver, symbol Ag, has a Face Centered Cubic structure and Silver color. Silver is a Transition Metal element. It is part of group 11 (copper family). Know everything about Silver Facts, Physical Properties, Chemical Properties, Electronic . An important point is that only a limited number of orbital shapes is possible for each value of n. If n = 1, then only the spherical 1s orbital is possible. When n is increased to 2, though, two orbital types (2s and 2p) become possible. Thus along with the 2s orbital, 3 other orbitals exist when n=2; 2p x, 2p y, and 2p z.

The electron configuration for phosphorus is 1s 2 2s 2 2p6 3 s2 3p3 and the orbital diagram is drawn below. 1.4: Electron Configurations and Electronic Orbital Diagrams (Review) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom indicates the number of valence . The electronic configuration of periodic elements shows the total number of electrons arranged in their atomic orbital. Let us see the electronic configuration of Ag. Electronic configuration of Ag is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 1. Silver is the transition metal atom its symbol is Ag. It is the 47 th periodic table element, . This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid .orbital notation of silverThe ratio of the average mass per atom of an isotope to 1/12 the mass of a carbon-12 atom. Relative atomic mass is also known as atomic weight (symbol: A r ). m: Silver (Ag) has an atomic mass of 47. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
elements in orbital notation, orbital notation with arrows and in short hand noble gas notation. a) Beryllium Orbital notation: 1s22s . Silver Orbital notation: 1s222s262p6 13s 103p 4s 3d10 4p6 5s 4d Orbital notation + Arrows: Noble gas notation:[Kr]105s1 4d 7. Which ground state elements correspond to the following electron configurations?That is, the orbital notation of indium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 1. What is Hund’s principle? Hund’s principle is a rule that helps to determine how electrons are distributed in orbitals when . Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which .An orbital is a wave function for an electron defined by the three quantum numbers, n, ℓ and m ℓ. Orbitals define regions in space where you are likely to find electrons. s orbitals (ℓ = 0) are spherical shaped. p orbitals (ℓ = 1) are dumb-bell shaped. The three possible p orbitals are always perpendicular to each other. Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first.
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Silver (Ag): Silver is a d-block element having atomic number 47. The group number and period number of Silver are 11 and 5 respectively.
Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n – 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum .
By following a specific set of steps, you can determine the electron configuration notation or create an electron orbital diagram for silver. Let’s explore the process: Steps to Write Electron Configuration for Silver. Step 1: Start by filling the orbitals in increasing energy order. Begin with the 1s orbital, which can hold a maximum of 2 .
Watch Pinay Katorse X porn videos for free, here on Pornhub.com. Discover the growing collection of high quality Most Relevant XXX movies and clips. No other sex tube is more popular and features more Pinay Katorse X scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you own.
orbital notation of silver|Iba pa